
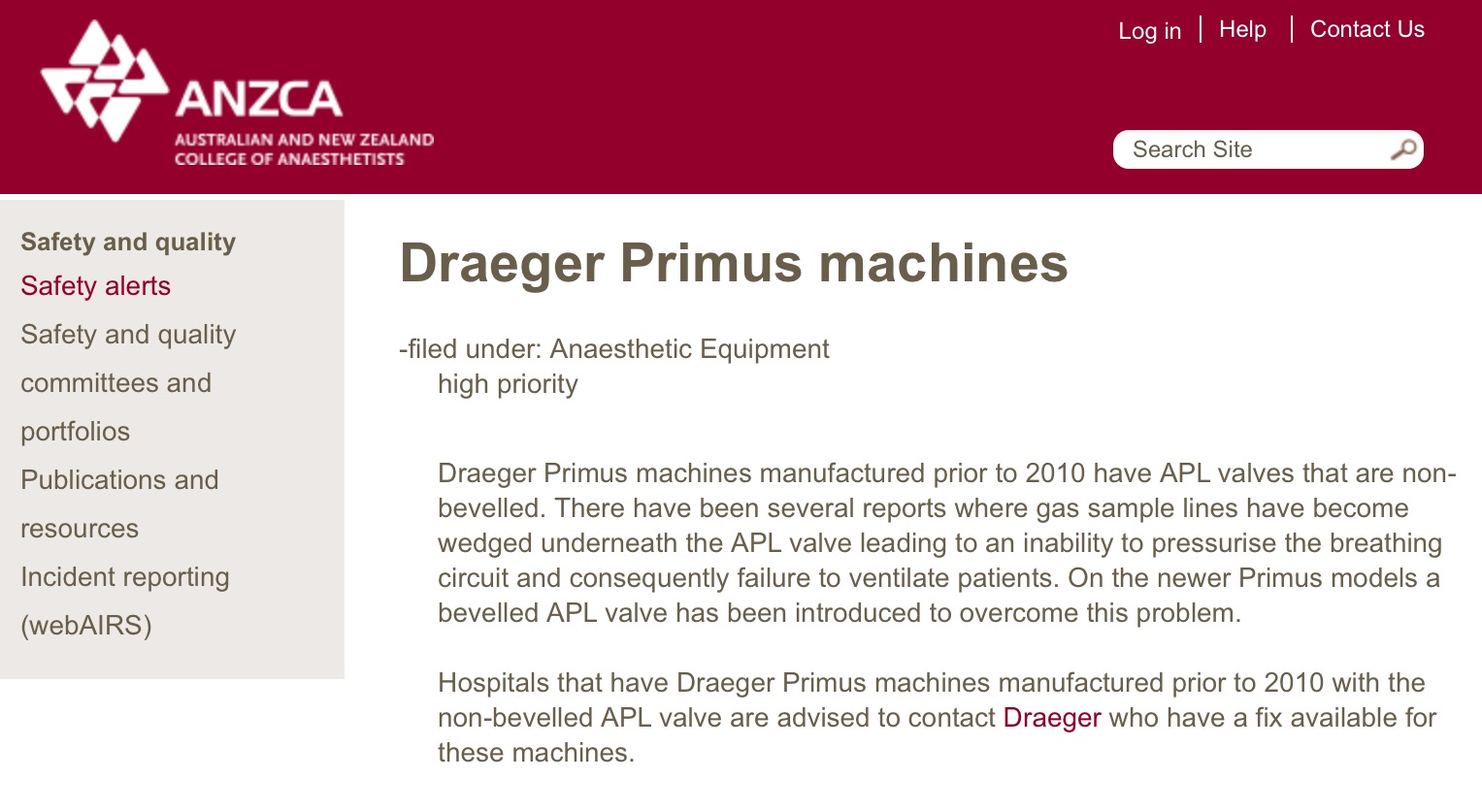
The Draeger anaesthetic machine has a poorly understood issue with its APL valve. When lifted it is actually open – even though it appears closed – creating an inability to pressurise the breathing circuit.
There are numerous reports of adverse events related to this issue. A redesign of the valve in 2010 to prevent the problem can be retrofitted onto older machines. We should ensure this is done for patient safety.
For more information click here.
On 17th March 2017 the TGA informed us that within Australia all old valves have now been replaced.

We recognise that this is an issue not just confined to Australia and have asked Draeger’s CEO to ensure replacement elsewhere.
Please sign the petition to recall old Draeger APL valves here.
The Draeger anaesthetic machine has a poorly understood issue with its APL valve. When lifted it is actually open – even though it appears closed – creating an inability to pressurise the breathing circuit.

It can be jammed open (see valve on right above) particularly when the gas sample line becomes trapped underneath. In turn patients can’t be ventilated – anaesthetists are often unable to diagnose the cause placing patients at unnecessary risk.
Draeger know about this problem and have introduced a bevelled APL valve on newer anaesthetic machines (see valve on right below):

There are numerous anaesthetic machines being used with the old hazardous APL valve. The new beveled APL valve can be retro-fixed onto older machines – we should ensure this is done.
Sign the petition to promote the recall of old Draeger APL valves.
Numerous episodes of APL trapping have been reported in the literature:
1. Gas leak related to Draeger Primus Anaesthetic Machine. Anaesthesia, July 2010.
3. Wires block APL valve interfering with ventilation. APSF newsletter Summer 2011.
5. Draeger APL valve. FDA – Adverse Event Report. Sept 2012.
7. Temporary malfunction of an APL valve. BJA, March 2006.
9. Failure to Ventilate with the Drager Apollo Anesthesia Workstation. Anesthesiology, March 2011.
10. Apollo Anesthesia Workplace. FDA – Adverse Event Report. Sept 2012.
12. Drager Apollo Anesthesia Workstation APL Valve on Workstation. FDA Adverse Event Report. Sept 2014.
14. A Malfunction of the APL Valve (in German). Anästh Intensivmed, Feb 2011.
16. Page 29 of Anaesthesia News. June 2012.
17. MAUDE report (despite sample line being clipped away) Sept 2012
In all of these cases the reporting anaesthetist is caught unaware – this is despite alerts (see below) demonstrating the inefficiency of alerts on their own as a safety solution. Still many anaesthetists are unaware of this problem.
The function of the APL valve being open when elevated is unique to Draeger machines. It is not a function intuitive to the majority of anaesthetists.
The case reports above represent only a fraction of the actual events. The author is aware of numerous other cases not reported here.
Several similar events have been reported to the FDA. We’re suspicious they may be due to APL trapping – there’s an inability to pressurise the circuit with manual ventilation mode on induction, in some instances being able to with mechanical modes which bypass the APL valve.
1. Draeger Medical AG KGAA Anesthesia Machine. Adverse Event Report. July 2006.
2. Primus Anaesthesia Unit. Permanent brain injury. Adverse Event Report. April 2013.
5. Patient became hypoxic, hypotensive and bradycardic. July 2011.
6. ‘The user almost lost the patient’. February 2009.
8. Significant circuit leak. January 2013.
They would cost Draeger approximately $350 each to replace.
We should ensure these old APL valves are replaced in the interest of patient safety.
Sign the petition to promote the recall of old Draeger APL valves.



Sign the petition to promote the recall of old Draeger APL valves.
